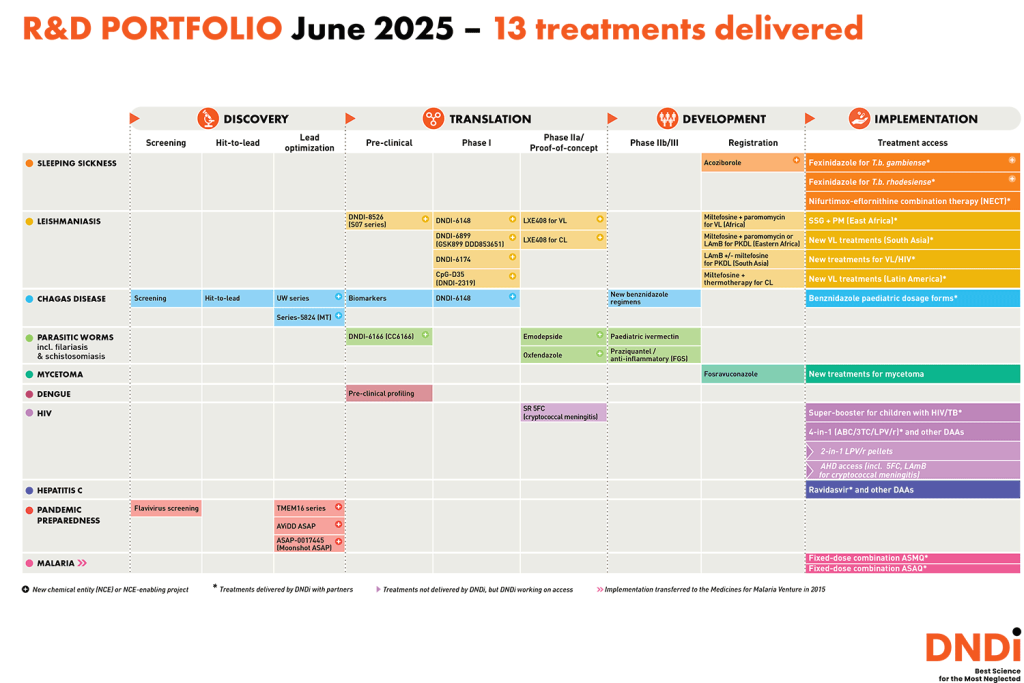
DNDi invests in bringing all-new treatments through the research and development (R&D) pipeline – both by repurposing or modifying drugs from other therapeutic areas and by identifying and developing entirely new chemical entities.
We are committed to developing safe, effective, and affordable treatments for people with neglected diseases and neglected patients. Together with our partners, we are working on over 40 projects, including 21 projects focused on identifying or developing new chemical entities. We are also running 16 clinical trials.
Making medical history for neglected patients
We develop urgently needed treatments for neglected patients and ensure they’re affordable, available, and adapted to the communities who need them.
Help neglected patients
DNDi’s generous supporters make sure we can take on the devastating diseases affecting the people who need our help the most.
Treatments delivered
Our teams have been working since 2003 to address gaps in the traditional biomedical R&D system that lead to serious unmet medical needs for neglected patients. Acting as a ‘conductor of a virtual orchestra’, DNDi collaborates with research partners around the world at all stages of the R&D process.
Together, we’ve discovered, developed, and delivered new treatments that reduce illness, suffering, and death from some of the world’s most neglected diseases. Discover more about the treatments we’ve delivered on our international website dndi.org.
TREATMENT DELIVERED
Fexinidazole for T.b. gambiense
An all-new, all-oral patient-friendly treatment. Approved by the European Medicines Agency in 2018, DNDi’s first ‘new chemical entity’ cures the most common form of sleeping sickness in just 10 days of simple, 1-pill-per-day treatment.
TREATMENT DELIVERED
4-in-1 (ABC/3TC/LPV/r)
A strawberry-flavoured, ‘4-in-1’ treatment for children. The 4-in-1 meets the treatment needs of the youngest children living with HIV. The combination of four antiretrovirals in easy-to-administer, heat-stable, strawberry-flavoured granules has been approved in South Africa.